There are so many articles out there by now about the Flint, Michigan, water issue, I debated adding mine to the list. But I noticed there are some interesting aspects about what has happened that I've not seen discussed elsewhere. So I decided to go ahead and throw my voice in with the others. Also, I think it is important that someone from the industry point out what all of us know, but the public and media may not which is that in general,
…We are all Flint, Michigan.
I'm going to discuss this point first just because the other topics I want to touch on involve more technical information that probably won't interest most people. So why do I say we are all Flint, Michigan? First a short history of lead in the water industry:
In many countries throughout the world, including the United States, lead pipes were used for centuries to convey water.
As you can see below, lead lines were used at the Roman Baths which were built in 70 A.D. in Bath, England.

So how did lead move from a commonly accepted material for water lines to the "do not use" list? Based on information in an article "The Lead Industry and Lead Water Pipes 'A Modest Campaign'" by Richard Rabin, MSPH, people started realizing in the late 1800s that maybe using lead for moving water around wasn't such a great idea. But even this knowledge didn't seem to immediately stop the use of lead for many more decades. According to the article:
"Although most cities in the United States were moving away from lead water pipes by the 1920s, it appears that this trend was not universal. National model plumbing codes approved lead into the 1970s and 1980s, and most water systems based their regulations on those codes. Federal guidelines and specifications also sanctioned lead pipes at least into the 1950s."
Lead use in water systems was not banned until passage of the Safe Drinking Water Act which in Section 1417 prohibited the:
“use of any pipe, any pipe or plumbing fitting or fixture, any solder, or any flux, after June 1986, in the installation or repair of (i) any public water system; or (ii) any plumbing in a residential or non-residential facility providing water for human consumption, that is not lead free.”
Later, in 1991, the United States Environmental Protection Agency (EPA) published the Lead and Copper Rule – a regulation to control lead and copper in drinking water. This rule established the action level (AL) of 0.015 mg/L for Pb and 1.3 mg/L for Cu based on 90th percentile level of tap water samples. It's interesting to note that this rule also states an exceedance of the AL is not a violation but "can trigger other requirements that include water quality parameter (WQP) monitoring, corrosion control treatment (CCT), source water monitoring/treatment, public education, and lead service line replacement (LSLR)."
If you are wondering why it took so long to get lead banned as a material in the water system, you can read Rabin's article linked above. My point I'm making here is that lead was commonly used all over the place. Over the 30 plus years I've been designing, building, and helping operate water systems, I've come across a lot of lead lines used for water services – the pipes carrying water from the public water main to people's homes and businesses. And because lead was such a common material, I never noticed lead only used for lines running to homes in low income areas. I've also seen lead lines running to the homes of the wealthiest in the community and to all types of businesses.
What really makes the difference on what type of service line someone has is when it was installed.
If it was put in decades ago before copper became the preferred choice, then the line is probably lead. In Werner Troesken's book, The Great Lead Water Pipe Disaster, he lists on page 11 in Table 1.1 50 of the major cities in the U.S. and the material used for service lines in the late 1900s for 46 of these cities. He notes 85% of the cities were using lead water pipes. Detroit, Michigan, is one of them, and the locations extend across our nation from Washington, D.C., to San Francisco, Calif. For all I know the White House might still have a lead water line.
 So if lead water lines are everywhere, just like in Flint, you might ask, how come we haven't dug up all these lines and replaced them? Well, replacing every lead line was definitely an option every community water supply regulated by the Lead and Copper rule could consider. However, I haven't heard of many that chose that alternative. Probably because the cost and disruption to the community is potentially tremendous and not cost effective compared to other solutions. Also, in many communities, ordinances place ownership of these water service lines with the property owner, so few communities wanted to have to go to their water customers and force them to put in new water lines. And even if the water supplier chose to pay for everything, the cost would ultimately be passed along anyway to customers through higher rates, and the property owner would still need to go through the disruption of having their yard torn up. So many community water supplies chose an alternative which involves adding chemicals like polyphosphates to the water to coat the pipes and prevent the leaching of lead and copper into the water. For most, it's really the most cost effective solution, and as you can see from the photo here, it's a fairly simple setup.
So if lead water lines are everywhere, just like in Flint, you might ask, how come we haven't dug up all these lines and replaced them? Well, replacing every lead line was definitely an option every community water supply regulated by the Lead and Copper rule could consider. However, I haven't heard of many that chose that alternative. Probably because the cost and disruption to the community is potentially tremendous and not cost effective compared to other solutions. Also, in many communities, ordinances place ownership of these water service lines with the property owner, so few communities wanted to have to go to their water customers and force them to put in new water lines. And even if the water supplier chose to pay for everything, the cost would ultimately be passed along anyway to customers through higher rates, and the property owner would still need to go through the disruption of having their yard torn up. So many community water supplies chose an alternative which involves adding chemicals like polyphosphates to the water to coat the pipes and prevent the leaching of lead and copper into the water. For most, it's really the most cost effective solution, and as you can see from the photo here, it's a fairly simple setup.
So if we are like Flint, are we too at risk?
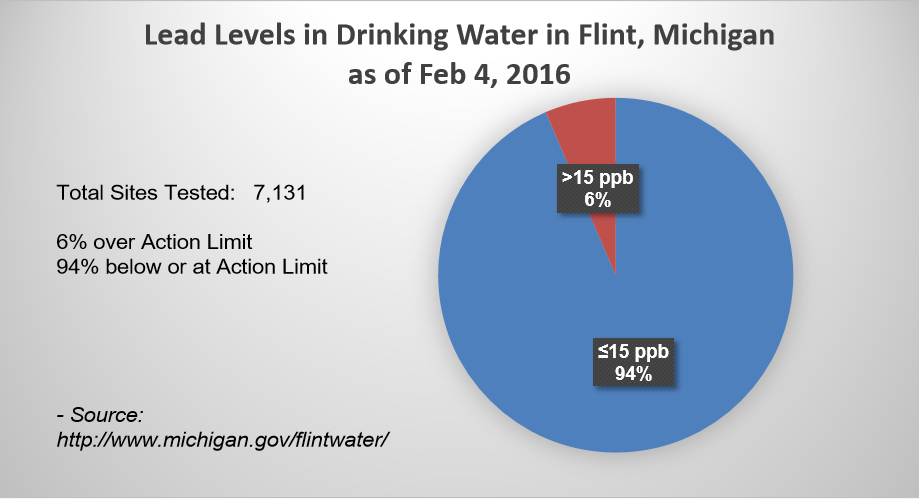
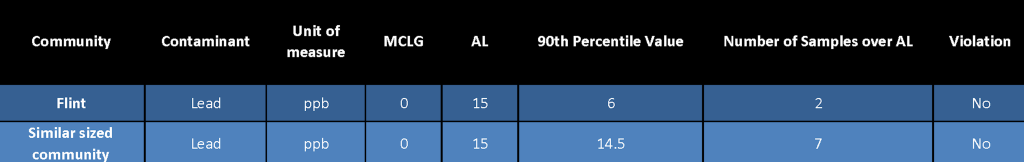
While I said all that to try to let people know Flint is not different than most other cities across our nation with respect to its water piping, it is important to remember, not everyone, including everyone in Flint, is at risk. Based on the lead and copper water testing results I saw from Flint, it seems the results showed a little over 10% of the sites tested over the Action Limit which was similar to results from the supply systems for which I worked. So for those in Flint, this would mean if they do not have a lead service line, they are probably at a low risk of having lead levels above the action level. And for those of you who do not live in Flint and have a lead water line, if your community water supply is in compliance with the Lead and Copper rule, levels of lead in your water would probably not exceed the action levels noted above. However, to be sure, you could always contact your water supplier and ask them to test your water for lead and copper.
Also, be cautious of blaming drinking water for lead in children when it is more likely to be lead paint
 If your community is going to analyze the lead levels in children and compare them over a specific time frame to determine if there is a lead problem, keep in mind the primary source of lead in children is not from drinking water, but from lead paint. So a true study would develop methodology that includes an analysis of each child's home environment over that time period. I tried to find the study or report from the pediatrician in Flint who analyzed the levels of lead in children in the area, but could not find any document summarizing her methodology, analysis, and testing to see if she had taken into account exposure to lead paint. Particularly if she has made a correlation between elevated levels and income levels of children who test positive for lead, I thought it would seem more likely lead paint would be the cause of that than would water. Not having found anything verifying she used the proper methodology, I have to wonder if there is a lead problem in Flint that will not be solved by addressing the drinking water because it is actually a lead paint problem. As the leadfreekids.org site states, "just a few particles of dust from lead-based paint are enough to poison a child."
If your community is going to analyze the lead levels in children and compare them over a specific time frame to determine if there is a lead problem, keep in mind the primary source of lead in children is not from drinking water, but from lead paint. So a true study would develop methodology that includes an analysis of each child's home environment over that time period. I tried to find the study or report from the pediatrician in Flint who analyzed the levels of lead in children in the area, but could not find any document summarizing her methodology, analysis, and testing to see if she had taken into account exposure to lead paint. Particularly if she has made a correlation between elevated levels and income levels of children who test positive for lead, I thought it would seem more likely lead paint would be the cause of that than would water. Not having found anything verifying she used the proper methodology, I have to wonder if there is a lead problem in Flint that will not be solved by addressing the drinking water because it is actually a lead paint problem. As the leadfreekids.org site states, "just a few particles of dust from lead-based paint are enough to poison a child."
And now for the technical discussion…
People familiar with the Flint story probably won't be surprised to hear that one of the main problems we had with a plant where I used to work was that the engineer who designed it based the treatment design on the parameters of the existing water supply. For almost a hundred years, the community had drawn water from a shallow acquifer lying under about seven acres of land. As part of the water plant construction, the city had decided to expand their supply by digging a new well. This well was located outside of the immediate area of the historic well field, but because it was in the same acquifer, the engineer assumed the water quality would be the same. First lesson I learned from this project:
Always test the water quality parameters of a new water supply – preferably before committing to its use
The other wells had about 0.5 parts of manganese while this new well had 3 parts iron. Now if you are in the industry, you can imagine how the plant performed if it was designed to treat 0.5 parts of manganese and now had to also oxidize 3 parts iron. Iron oxidizes first so when that new well was running, there wasn't enough ozone, which we were using as the oxidant, to oxidize everything. Guess what we had to do? We pre-chlorinated to assist in the oxidation. And guess what the extra chlorine added ahead of the ozonators did? Increased the corrosiveness of the water.
So it seems like Flint built their new plant because they had been receiving treated water from Detroit then decided to enter into an agreement with other communities to pull water from the lake. And what I am wondering is did the plant designer set up the treatment system for the lake water without knowing or ignoring the river water would be used in the interim? If that was the case and the water quality is different between the two sources, this could be causing many of the issues they are having with corrosion. If changes had to be made to the process to address the change in the quality of the water supply, there could be impacts to the finished water quality that were not anticipated in the design.
Another issue with a change in water supply is that it can change the anticipated pH for which the plant was designed. And pH definitely has an impact on the performance of the treatment process. If pH is too low, the water can be more corrosive; if pH is too high, certain substances like calcium carbonate can fall out of solution, bind to the pipes, and reduce flow. Below is the inside of a pipe in which substances came out of solution and adhered to the pipe.

I also looked at the treatment reports for Flint's water plant to see if there seemed to be anything unusual with their process that could affect the corrosiveness of the water. This is important to look into because replacing water lines may lower the risk of lead in drinking water supplying homes and businesses, but it will not improve the water quality. One thing I noticed is that according to Flint's Consumer Confidence Reports for 2014, they are using ozone for taste and odor control and as a pretreatment disinfection. Because of my past experience I wondered if their use of ozone was impacting the corrosiveness of their water.
In our system, which used ozone as an oxidant (a photo of one of our ozonators is shown below) and required the need to pre-chlorinate, we initially experienced the formation of nitrates/nitrites. The level never exceeded the regulated amount, but it still caused problems in the system. Unfortunately we had to treat the problem with more chlorine until the nitrates/nitrite formation was under control. Fortunately for Flint, it doesn't appear this is an issue, but it is something to be on the lookout for.

We also noticed that in addition to the corrosive water causing a change in the level of lead and copper from the levels tested prior to the plant going online, there were many failures of metal-based parts in equipment exposed to water. In particular, the impellers of our pumps and the pumps in the fire trucks were degraded to the point they had to be replaced. I am not sure if Flint has experience any of this, but it is also something to monitor. Fortunately the addition of the phosphates and the addition of chemicals to increase our pH slightly seemed to elminate this problem for us.
Another issue that came up was raised by industries in our city that relied upon our water for their operation. A change in water quality can make a significant difference for these companies not only in the successful operation of their own process, but in their costs. I suspect this became an issue in Flint because I read a report indicating one of the industries had to be supplied with different water. Changing lead lines will not address this issue. Instead the treatment system needs to be analyzed and the water quality addressed.
In the end, what all of the problems at our plant taught me was that the design and operation of a water treatment process is highly dependent on a strong knowledge of water chemistry. And over the years when I've talked with operators starting up new plants, I've found they are all struggling to solve the same issues again and again. And it all seems to be caused by the designer ignoring water chemistry. Engineers are typically the people designing water plants, but most engineers are not chemists. Which has always made me wonder
Why doesn't the EPA require a water chemist to sign off on new plant designs?
Information about the source water used in the design, the use of ozone, the pH and perhaps even temperature of the water, and the apparent use of high amounts of chlorine could indicate something might not be right with the treatment process chosen or implemented in Flint. And while I cannot say without a doubt that these are the issues impacting the corrosiveness of Flint's water, they are clues that tell me someone who knows water chemistry should be analyzing their treatment process. Because the government can throw hundreds of millions of dollars at this and everyone's pipes can be replaced, but in the end, the root of the problem might not be addressed which could be the use of the wrong treatment process for that particular water supply.
 Having worked for many years in the water industry, I've closely followed the recent events related to the lead controversy in Flint, Michigan. If you're a regular reader of this blog, you've also probably seen the articles my partner and I posted about the topic. As we continued to discuss the topic over the last few months, we realized we could continue to offer through our blog information to people about minimizing their exposure to lead in their homes. But what could we do to help people be sure they are not exposed to lead once they ventured outside their home? So we started to wonder if it might be useful to have a place online where people could check to see the levels of lead in places they were thinking of visiting. Today we are launching that site – Water Unleaded – to serve as a type of drinking water quality registry focusing on the content of lead in drinking water.
Having worked for many years in the water industry, I've closely followed the recent events related to the lead controversy in Flint, Michigan. If you're a regular reader of this blog, you've also probably seen the articles my partner and I posted about the topic. As we continued to discuss the topic over the last few months, we realized we could continue to offer through our blog information to people about minimizing their exposure to lead in their homes. But what could we do to help people be sure they are not exposed to lead once they ventured outside their home? So we started to wonder if it might be useful to have a place online where people could check to see the levels of lead in places they were thinking of visiting. Today we are launching that site – Water Unleaded – to serve as a type of drinking water quality registry focusing on the content of lead in drinking water.